In November 2018, we received FDA approval of Sympazan (clobazam) oral film. Sympazan is a film formulation of clobazam, a benzodiazepine used as an adjunctive therapy for seizures associated with Lennox-Gastaut syndrome (LGS).
Sympazan, with its patented PharmFilm® technology delivery system, is an innovative choice for caregivers and patients. Sympazan is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older.
For full prescribing information, click here.


The Sympazan Savings Card can help lower your out-of-pocket costs, if you’re an eligible patient with commercial insurance.
Depending on your insurance coverage, you may pay as little as $10 for a 30-day prescription of Sympazan. Please see Program Terms, Conditions, and Eligibility Criteria

Subject to eligibility. Restrictions apply.
See eligibility, terms, and conditions
What is the most important information I should know about SYMPAZAN?
Do not take SYMPAZAN if you are allergic to clobazam or any of the ingredients in SYMPAZAN.
Before you take SYMPAZAN, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking SYMPAZAN with certain other medicines can cause side effects or affect how well SYMPAZAN or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
The most common side effects of SYMPAZAN are: sleepiness; pain with urination; slurred speech; drooling; fever; tiredness; constipation; acting aggressive; angry; or violent; problems with breathing; cough; and difficulty sleeping. These are not all the possible side effects of SYMPAZAN. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
SYMPAZAN is a prescription medicine used along with other medicines to treat seizures associated with Lennox-Gastaut Syndrome in people 2 years of age or older. It is not known if SYMPAZAN is safe and effective in children less than 2 years old.
Please see full Prescribing Information, including Medication Guide with important warning.
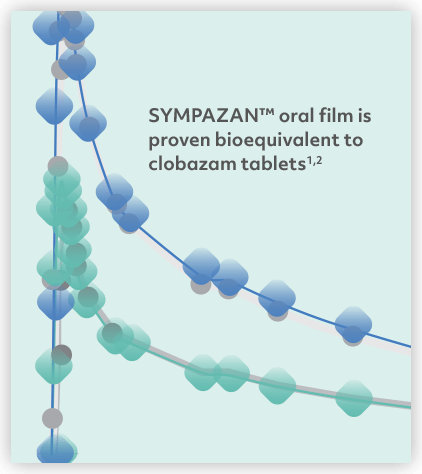
1. Integrated Clinical and Statistical Report. A pivotal, open-label, randomized, single-dose, four-period, four-arm, crossover, comparative bioavailability study of clobazam 20 mg and 10 mg oral films and ONFI® 20 mg and 10 mg tablets in healthy male and female volunteers under fasting conditions. August 2017.
2. SYMPAZAN [package insert]. Warren, NJ: Aquestive Therapeutics; November 2018.